About VTUEEE 2021
VTUEEE or Vel Tech Undergraduate Engineering Entrance Examination is a university level entrance exam holds by Vel Tech Rangarajan Dr. Sagunthala R and D Institute of Science and Technology for offering admissions in B.Tech available in the institute. Vel Tech Technical University is considered to be one of the premium universities in India and sees lakhs of students applying for admission every year. Engineering at VTU remains one of the most sought-after courses.
VTUEEE is a computer-based online examination that consists of 90 questions, divided into three sections, i.e., Physics, Chemistry, and Mathematics. Aspiring candidates must have to complete the exam within a time duration of 3 hours.
VTUEEE Exam 2021: Key Element
| ATTRIBUTES | DETAILS |
| Name | VTUEEE |
| Full-Form | Vel Tech Undergraduate Engineering Entrance Examination |
| Conducting Authority | Vel Tech Rangarajan Dr. Sagunthala R and D Institute of Science and Technology |
| Exam Level | University level |
| Courses Offered | Undergraduate Engineering |
| Registration Mode | Online/Offline |
| Exam Mode | Centre Based Test (CBT) |
| Numbers of Questions | 90 |
| Exam Pattern | MCQ |
| Duration | 3 Hours |
| Language | English |
| Exam Website | https://www.veltech.edu.in/ |
VTUEEE Important Dates 2021
The official announcement for the 2021 entrance exam VTUEEE is made on the original site VTUEEE. Application for the exam can be done through both online or offline mode. Students can submit the application form online through the university's official site.
| VTUEEE 2021 Events | Phase 1 Dates | Phase 2 Dates |
| Release of VTUEEE application form | December 14, 2020 | December 14, 2020 |
| Last date to apply for VTUEEE 2021 | Slot
1 - February 17, 2021 Slot 2 - March 17, 2021 | Slot
1 - April 18, 2021 Slot 2 - May 9, 2021 Slot 3 - May 25, 2021 Slot 4 - May 31, 2021 |
| VTUEEE exam date 2021 | Slot
1: February 20 to 24, 2021 Slot 2: March 20 to 24 | Slot
1: April 21 to 25, 2021 Slot 2: May 12 to 16, 2021 Slot 3: May 29 to 31, 2021 Slot 4: June 4 to 6, 2021 |
| Declaration of VTUEEE result | May 16, 2021 | To be announced |
| Commencement of counselling | To be announced | To be announced |
VTUEEE Eligibility Criteria 2021
Vel Tech Rangarajan Dr. Sagunthala R and D Institute of Science and Technology set specific minimum eligibility criteria, which must be fulfilled for admission to respective engineering, and technology programs. Before applying for the VTUEEE the applicants are suggested to check the criteria of academic qualifications.
The conditions of eligibility for VTUEEE 2021 ar given below:
- A candidate seeking admission for the undergraduate courses must have passed class 10+2 or equivalent from a recognized board or university.
- Relaxation of 5% has been given to the reserved category (40%).
- Candidates must also clear the English with at least 30% marks.
- Compulsory Subjects: Chemistry, Physics, and Mathematics /Biology
- Nationality: Only Indian Nationals are eligible.
- Age Limit: 25 years of age as of July 1, 2021
- Students in Class 10+2 or awaiting board results are also eligible to apply for VTUEEE.
VTUEEE 2021 Application Process
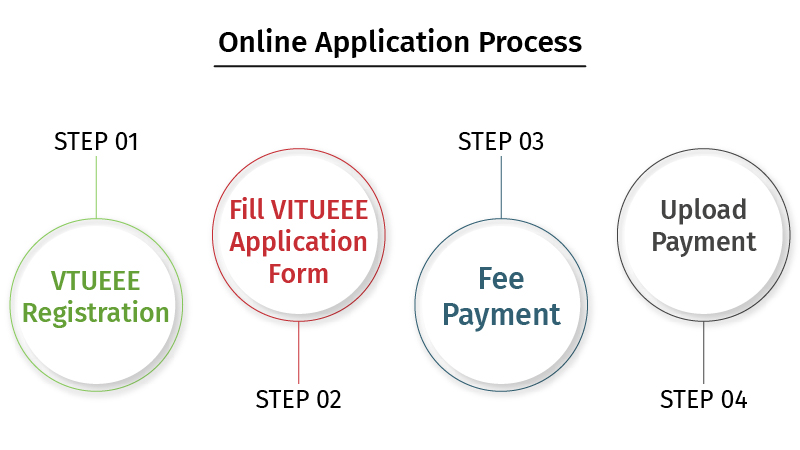
VTUEEE Exam 2021 registration process is done in both an online and offline mode. The registration commences on 14th December 2020. Candidates who want to participate in the exam have to apply for the exam by filling the application form through the official website of the VTUEEE that involves the following steps:
Online VTUEEE 2021 Form
Step 1: Registration
- Visit the official website of VTUEEE – Click Here
- Click on the ‘VTUEEE 2021 B.Tech Admission Open’. It will redirect you to the registration form.
- Fill required details.
- Click ‘Register’.
- Registration ID and Password will be sent to your registered email ID to login to the VTUEEE application form.
Step 2: Fill VTUEEE online application form 2021
- Re-login using unique VTUEEE Registration ID
- Enter all the required details such as the Candidate's Name, Parent’s Name, Date of Birth, Gender (Male/Female), Religion, Caste (General/OBC/SC/ST), Nationality, and so on.
Step 3: Fee Payment
- After clicking Save & Next, the fee payment page will be redirected.
- Choose payment mode (Net Banking, Credit card, and Debit Card).
- Click Proceed to pay.
Application Fee details for exam
| Group/Category | Application Fee (INR) |
| All | 950 INR |
Step 4: Upload Documents
- Upload the scanned photograph.
- Upload the scanned signature.
Step 5: Final Submission
- After completing all the steps mentioned above candidates will have to do the final submission of the VTUEEE application form.
- Don’t forget to download the filled-in application form.
Offline VTUEEE 2021 Form
Candidates can also apply for VTUEEE 2021 with a ₹ 950 demand draft drawn in favour of “VelTech Dr.RR & Dr.SR Technical University”, payable at Chennai, to The Director- UG Admission with a request letter containing the full address of the applicant and send it to the following address:
Director, UG Admission Vel Tech Dr. RR & Dr.SR Technical University
#42 Avadi-Vel Tech Road, Avadi, Chennai-600 062, Tamil Nadu, India.
Phone: 044-26841601,044-26840896/ 249
Toll Free Number: 180030706949
Email: admission@veltechuniv.edu.in
VTUEEE Exam Pattern 2021
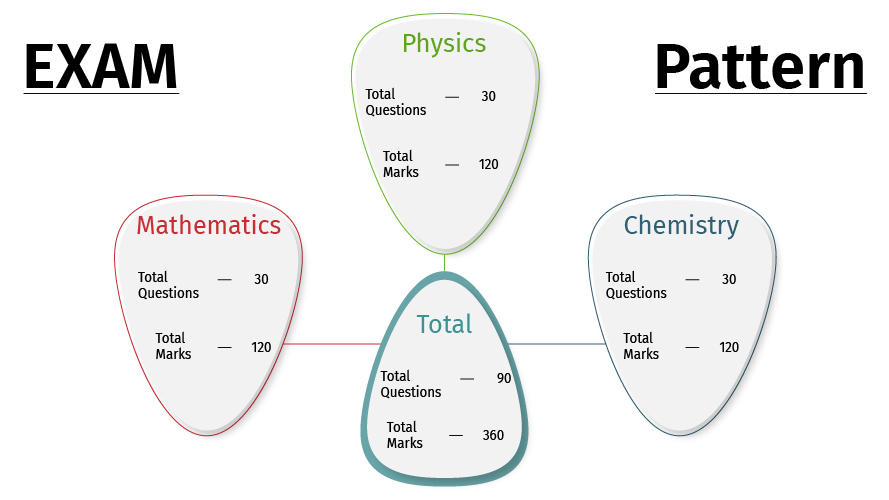
VTUEEE 2021exam is conducted in the Computer Based Test (CBT) mode. The VTUEEE paper consists of 90 questions that are divided into three sections, i.e., Mathematics, Physics, and Chemistry. They will have to answer all the questions within the time duration of 3 hours.
- VTUEEE 2021 will be conducted only in the online mode, i.e., CBT mode.
- The exam duration is of 3 hours.
- All the questions asked in the test will be related to the VTUEEE 2021 syllabus.
- As per the VTUEEE 2021 exam pattern, there will be Multiple Choice Questions
- Each correct answer will carry one mark.
- No marks will be deducted for incorrect answers.
| Sections | Total Questions | Total Marks |
| Physics | 30 | 120 |
| Chemistry | 30 | 120 |
| Mathematics | 30 | 120 |
| Total | 90 | 360 |
VTUEEE Exam Syllabus 2021
The syllabus for the VTUEEE 2021 is drafted as per the level of the Class 12 course. The paper is based on the top main subjects of Physics, Chemistry, and Mathematics for admission. Here are the course details as per the preceding entrance exam below:
VTUEEE 2021 Physics Syllabus
- Mechanics: Uniform and non-uniform motion, average speed and instantaneous velocity, uniformly accelerated motion, Scalar and vectors, relative velocity, Motion in a plane, projectile motion-uniform circular motion-force and inertia, Newton’s first law of motion-momentum, Newton’s second law of motion, impulse, Newton’s third law of motion-law of conservation of linear momentum and its applications, equilibrium of concurrent forces-static and kinetic friction, the law of friction, rolling friction, basic concepts of rotational motion-moment of a force-torque, angular momentum, conservation of angular momentum and its application momentum of inertia-radius of gyration, values of moments of inertia for simple geometrical objects, parallel and perpendicular axes theorems and their applications. Rigid body rotation, equations of rotational motion.
- Properties of matter: The universal law of gravitation, acceleration due to gravity, variation of ‘g’ with altitude, latitude, and depth, gravitation potential – escape velocity and orbital velocity, geostationary satellites, Kepler’s laws of planetary motion. Solids, elastic behavior, stress-strain, Hooke’s law, Moduli of elasticity, the relation between them, surface tension, capillarity, applications, viscosity, Poiseuille’s formula, stokes law, applications, streamline and turbulent flow, Reynolds number, Bernoulli’s theorem, application
- Heat and thermodynamics: Equation of state of a perfect gas, work done on compressing a gas, Kinetic theory of gases, assumptions, the concept of pressure, Kinetic energy and temperature, RMS speed of gas molecules, degrees of freedom, the law of equipartition of energy, applications to specific heat capacities of gases, mean free path, Avogadro’s number. Thermal Equilibrium, zeroth law of thermodynamics, the concept of temperature, heat, work and internal energy, the first law of thermodynamics, Second law of thermodynamics, reversible and irreversible processes, Carnot engine and its efficiency.
- Optics: Reflection of light, spherical mirrors, mirror formula, Refraction of light, total internal reflection and its applications, refraction at spherical surfaces, lenses, thin lens formula, lens maker’s formula, Magnification, power of a lens, a combination of thin lenses in contact, a combination of a lens and a mirror, refraction, and dispersion of light through a prism, Scattering of light, the blue color of the sky and reddish appearances of the sun at sunrise and sunset Wavefront and Huygens’s principle, laws of reflection and refraction using Huygens’s principle, interference, young’s double-slit experiment and expression for fringe width, diffraction due to a slit, the width of central maximum, resolving power of microscope and astronomical telescope, polarized light, Brewster’s law, uses of plane polarized light and Polaroid’s.
- Electricity: Electric Current, the flow of charges in a metallic conductor, drift velocity and mobility and their relation with electric current, Ohm’s law, electrical resistance, V-I characteristics, electrical resistivity and conductivity, classification of materials in terms of conductivity, Carbon resistors, colour code for carbon resistors, a combination of resistors, series and parallel, the temperature dependence of resistance, the internal resistance of a cell, potential difference and emf of a cell, combinations of cells in series and in parallel, Kirchoff’s law, illustration by simple circuits, Wheatstone’s Bridge and its application for temperature coefficient of resistance measurement, meter bridge, a special case of Wheatstone bridge, potentiometer principle, comparing the emf of two cells. Electromagnetic induction, Faraday’s law, induced emf and current, Lenz’s law, Self-induction, Mutual induction, self-inductance of a long solenoid, the mutual inductance of two long solenoids, Methods of inducing emf - (i) by changing magnetic induction (ii) by changing area enclosed by the coil and (iii) by changing the orientation of the coil (quantitative treatment), AC generator, commercial generator, (Single phase, three phases), Eddy current, applications, transformer, long distance transmission, alternating current, measurement of AC, AC circuit with resistance, AC circuit with an inductor, AC circuit with a capacitor, LCR series resonance circuit, Q factor, power in AC circuits.
- Magnetism: Biot – Savart law and its application to current carrying circular loop, Ampere’s law and its applications to infinitely long current carrying straight wire and solenoid, Force on a moving charge in uniform magnetic and electric fields, cyclotron Force on a current-carrying conductor in a uniform magnetic field, the force between two parallel current-carrying conductors, the definition of ampere, torque experienced by a current loop in a uniform magnetic field, moving coil galvanometer, its current sensitivity and conversion to ammeter and voltmeter. Current loop as a magnetic dipole and its magnetic dipole moment, Bar magnet as an equivalent solenoid, magnetic field lines, earth’s magnetic field and magnetic elements, para, dia, and ferromagnetic substances Magnetic susceptibility and permeability, hysteresis, electromagnets, and permanent magnets.
- Atomic physics: Atomic structure, the discovery of the electron, specific charge (Thomson’s method) and charge of the electron (Millikan’s oil drop method), alpha scattering, Rutherford’s atom model, Bohr’s model, energy quantization, energy, and wavenumber expressions, Hydrogen spectrum, energy level diagrams, sodium and mercury spectra, excitation and ionization potentials. Dual nature of radiation, photoelectric effect, Hertz and Lenard’s observations, Einstein’s photoelectric equation, particle nature of light, matter wave, wave nature of particle de Broglie relation, Davisson-Germer experiment.
- Nuclear physics: Nuclear properties, nuclear radii, masses, binding energy, density, charge, isotopes, isobars and isotones, nuclear mass defect, binding energy, stability of nuclei, Bainbridge mass spectrometer, Nature of nuclear forces, Neutron and its discovery Radioactivity, alpha, beta and gamma rays and their properties, radioactivity decay law, mass energy relation, mass defects, the binding energy per nucleon and its variation with mass number, nuclear fission, and nuclear fusion.
- Electronic devices: Semiconductors, semiconductor - diode, I-V characteristics in forward and reverse bias, diode as rectifier, I-V characteristics of LED, photodiode, solar cell and Zener diode, zener diode as a voltage regulator, junction transistor, transistors action, characteristics of the transistor, transistor as an amplifier (common emitter configuration) and oscillator. Logic VTUEEEs (OR, AND, NOT, NAND and NOR) transistor as a switch.
- Laser and optic fiber: Interaction of radiation with matter, Essentials of Laser, Types of Laser - Ruby Laser, He-Ne Laser, Semiconductor Laser, Application of Lasers, Optical Fibers – Propagation of light through an optical fiber, Modes of Propagation, Types of optical fibers, Optical fiber communication system, Attenuation in fibers.
VTUEEE 2021 Chemistry Syllabus
- Some basic concepts in chemistry: Matter and its nature, Dalton’s Atomic theory, Concept of Atom, Molecule, element, and compound, Laws of chemical combination, Atomic and Molecular masses, Mole Concept, Molar mass, chemical equations, Stoichiometry.
- S Block, P Block, d block, f block elements: Group 1 and 2 elements, Electronic configuration, General methods in physical and Chemical properties of elements, anomalous properties of the first element of each group, diagonal relationship P Block elements, Electronic configuration, General trends in physical and chemical properties of elements across the periods and down the groups, unique behavior of the first element in each group. Structure, properties, and uses of allotropes and oxides of Carbon, Silicon tetrachloride, Silicates, Zeolites and Silicones, Allotropic forms of Phosphorous, Structures of oxides and Oxoacids of nitrogen and phosphorous, Structures of oxoacids of Sulphur, Trends in the acidic nature of hydrogen halides, Structures of inter halogen compounds and oxides and oxo acids of halogen D and f block elements, electronic configuration, general trends in properties of the first row transition elements, ionization enthalpy, oxidation states, atomic radii, color, Catalytic behavior, magnetic properties, complex formation, interstitial compounds, Lanthanides: electronic configuration, oxidation states Actinides: Electronic configuration, oxidation states.
- Co-ordination Chemistry and Solid state Chemistry: IUPAC nomenclature of mononuclear coordination compounds, Isomerism, Geometrical isomerism in 4 Coordinate, 6 coordinate complexes, Theories of Coordination compounds-Werner’s theory, Valence bond theory Lattice- unit cell, systems, types of crystals, packing in solids, ionic crystals, Imperfections in solids, point defects, X-ray diffraction, Electrical property, Amorphous, Crystalline
- Thermodynamics, chemical equilibrium, and chemical kinetics: I and II law of thermodynamics, Spontaneous and non-spontaneous processes Entropy, Gibb’s free energy, Free energy change, and chemical equilibrium, Law of mass Action, Lechattlier’s principle, applications of chemical equilibrium, Rate expression Order and Molecularity of reactions, Zero order, first order and Pseudo first order Reaction, half-life period, Arrehenius equation activation energy
- Redox reactions and Electrochemistry: Electronic concepts of oxidation and reduction, redox reactions, oxidation number, rules for assigning oxidation number, balancing redox reactions. Electrolytic and metallic conduction, conductance in electrolytic solutions, molar Conductivities and their variation with concentration, Kohlrausch’s law, and its Applications. Electrochemical cells, Electrolytic and Galvanic cells, different types of electrodes, Electrode potentials including standard electrode potential, half cell and cell reactions Emf of a Galvanic cell and its measurement, Nernst equation, and its applications. Relationship between cell potential and Gibbs energy change, Dry cell, and lead Accumulator, Fuel cells.
- Purification and characterization of organic compounds: Purification- crystallization, sublimation, distillation, differential extraction, and Chromatography - principles and their applications. Quantitative analysis, detection of nitrogen, sulfur, phosphorous, and halogens, Estimation of carbon, hydrogen, nitrogen, halogens, sulfur, phosphorous. Calculations of empirical formulae, and molecular formulae, numerical problems in Organic quantitative analysis.
- Some basic principles of organic chemistry: Tetra valency of carbon, shapes of simple molecules, hybridization (s & p), classification of organic compounds based on functional groups, and those containing halogen Oxygen, nitrogen, and Sulfur, Homologous series, isomerism,-Structural and stereo Isomerism. Electronic displacement in a covalent bond,- inductive effect, electrometric effect, Resonance, and hyper conjugation, common types of organic reactions- substitution, Addition, elimination, and rearrangement.
- Hydrocarbons: Classification, isomerism, IUPAC nomenclature, general methods of preparation, properties, and reactions, Alkanes, conformations: sawhorse and Newman projections ( of ethane) Mechanism of electrophilic addition, the addition of hydrogen, halogens, water, hydrogen Halides, (Markownikoff’s and peroxide effect) ozonalysis and polymerization Alkynes- acidic character, addition of hydrogen, halogens, water and hydrogen halides Polymerization. Aromatic hydrocarbons - Nomenclature, benzene, structure, and automaticity, Mechanism of electrophilic substitution, halogenations, nitration, Friedel Craft’s Alkylation, and acylation, directive influence of the functional group in mono substituted Benzene.
- Organic compounds containing halogen: General methods of preparation, properties, and reactions, Nature of c-x bond, Mechanism of substitution reactions.
- Organic compounds containing Oxygen: Alcohols, Phenols and ethers, identification of primary, secondary, and tertiary Alcohols, mechanism of dehydration, acidic nature of phenols, electrophilic Substitution reactions, halogenations nitration, and sulphonation, Reimer TiemanbReaction, ethers- Structure Aldehydes and Ketones: Nature of carbonyl group, nucleophilic addition, > C = O group, relative reactivities of aldehydes, and ketones Important reactions such as –Nucleophilic addition Reactions (addition of HCN, NH3, and its derivatives) Grignard reagent, oxidation Reduction, acidity, Cannizzaro reaction, Haloform reaction, Chemical tests to distinguish between aldehydes and Ketones Organic compounds containing Nitrogen: General methods of preparation, properties, reactions and uses. Amines, nomenclature, classification, structure, basic character, and identification Of primary, secondary and tertiary amines and their basic character Diazonium salts-importance in synthetic organic chemistry.
- Bio molecules: Carbohydrates-classification, aldoses and ketones, monosaccharides (glucose And fructose), and constituent monosaccharides of oligosaccharides (sucrose, lactose, And maltose)Proteins-elementary idea of? –amino acids, peptide bond, polypeptide, proteins Primary, secondary, tertiary, and quaternary structure, denaturation of proteins, Enzymes Vitamins-classification, and functions.
- Polymers: Classification, general methods of polymerization- addition and condensation Copolymerization, Natural and synthetic rubber, vulcanization, some important polymers with emphasis on their monomers and uses-polythene, nylon, polyester, and backlite.
VTUEEE 2021 Mathematics Syllabus
- Partial fractions: Partial fractions of f(x)/g(x)when g(x) contains (i) non-repeated linear factors (ii) repeated and non-repeated linear factors; (iii) non-repeated irreducible quadratic factors; (iv) repeated and non-repeated irreducible quadratic factors.
- Complex numbers: Complex number as an ordered pair of reals; representation of complex numbers in the form a+ib and their representation in a plane; Argand diagram, algebra of complex numbers, modulus and argument (or amplitude) of a complex number; the square root of a complex number; triangle inequality.
- Quadratic equations: Quadratic equations with real coefficients and their solutions (roots); the relation between the roots and the coefficients; nature of roots; formation of quadratic equations with given roots; simple problems.
- Matrices and determinants: Matrices; algebra of matrices; types of matrices; symmetric and skew-symmetric matrices; orthogonal matrices; determinant of a square matrix; properties of determinants; evaluation of determinants; Test of consistency and solution of simultaneous linear equations in two and three unknowns.
- Trigonometry: Trigonometric identities; trigonometric functions and inverse trigonometric functions and their properties.
- Permutations and Combinations: Fundamental principles of counting; permutation as an arrangement and combination as a selection; the meaning of NPR and NCR simple problems.
- Binomial theorem for a positive integral index: Binomial theorem for a positive integral index; general term and middle term; binomial coefficients and their properties; simple problems.
- Two dimensional coordinate geometry: The cartesian system of rectangular co-ordinates in a plane; distance formula; section formula; locus and its equation; the slope of a straight line; parallel and perpendicular lines; intercepts of a line on coordinate axes. Various forms of the equation of a line; point of intersection of two lines, angle between lines; a distance of a point from a line; ortho centre, centroid, and circumcenter of a triangle; equation of the line passing through the point of intersection of two lines. Equation of a circle; radius and centre; points of intersection of a straight line and a circle; equation of a tangent; the condition for a line to be a tangent to a circle.
- Three-dimensional Geometry: Coordinates of a point in space; distance between two points; section formula; direction ratios and direction cosines; the angle between two intersecting lines; equations of a line and a plane in different forms.
- Vector algebra: Vectors and scalars; addition of vectors, components of a vector, scalar and vector products; the angle between two vectors; parallel vectors; perpendicular vectors; scalar and vector triple products; coplanar vectors; unit normal vector.
- Limit, continuity, and differentiability: Real-valued functions; graphs of simple functions; limit of a function; continuity of a function at a point and in an interval; differentiation of the sum, difference, product and quotient of two functions, differentiation of trigonometric, inverse trigonometric, logarithmic, exponential, composite and implicit functions; derivatives of order upto two; applications of derivatives; monotonic functions; maxima and minima of functions of one variable.
- Integration: Integral as anti-derivative; evaluation of integrals involving algebraic, trigonometric, exponential, and logarithmic functions; integration by substitution; integration by parts; integration by partial fractions; Bernoulli rule; definite integrals and their properties; simple problems.
- Ordinary differential equations: Order and degree; formation of differential equations; solution of differential equations by the method of separation of variables, solution of the homogeneous differential equation; solution of linear differential equation of the type dy/dx + P(x) y = Q(x).
- Set theory & Probability: Sets and their representations; union, intersection, and complements of sets; simple laws. Random experiment; sample space; event as a subset; Probability of an event; addition and multiplication theorems of probability; Bernoulli trials; Binomial distribution.
Courses Offered by VTUEEE 2021
VTUEEE offered B.Tech courses admission in Vel Tech Rangarajan Dr. Sagunthala R and D Institute of Science and Technology. Candidates can choose out of the following B.Tech specializations, which are as follows:
- B.Tech - Civil Engineering
- B.Tech - Mechanical Engineering
- B.Tech - Automobile Engineering
- B.Tech - Aeronautical Engineering
- B.Tech – Computer Science and Engineering
- B.Tech – Computer Science and Engg (Animation & Game Design)
- B.Tech – Computer Science and Engg (Data Analytics)
- B.Tech – Computer Science and Engg (Financial Computing)
- B.Tech – Computer Science and Engg (Networking & Cyber Security)
- B.Tech – Electrical and Electronics Engineering
- B.Tech – Electrical and Electronics Engineering (Energy Engineering)
- B.Tech – Electronics and Communication Engineering
- B.Tech – Electronics and Communication Engineering (Embedded systems)
- B.Tech – Electronics and Communication Engineering (Smart Systems)
- B.Tech – Information Technology
- B.Tech – Information Technology (Cloud Computing)
VTUEEE Exam Centres 2021
VTUEEE Exam 2021 is going to be held at 85 Exam Centres in 35 cities in India. Tabulated below is the list of exam centers with codes.
| State | Exam Centres |
| Tripura |
|
| Andaman & Nicobar |
|
| Andhra Pradesh |
|
| Arunachal Pradesh |
|
| Assam |
|
| Bihar |
|
| Chattisgarh |
|
| Delhi |
|
| Gujarat |
|
| Haryana |
|
| Jharkhand |
|
| Karnataka |
|
| Kerala |
|
| Madhya Pradesh |
|
| Maharashtra |
|
| Orissa |
|
| Puducherry |
|
| Punjab |
|
| Rajasthan |
|
| Tamil Nadu |
|
| Telangana |
|
| Uttar Pradesh |
|
| West Bengal |
|
| Goa |
|
| Himachal Pradesh |
|
| Jammu & Kashmir |
|
| Manipur |
|
| Meghalaya |
|
| Mizoram |
|
| Nagaland |
|
| Sikkim |
|
| Uttarakhand |
|
| Dadra & Nagar Haveli |
|
| Daman & Diu |
|
| Lakshadweep |
|
Tips for VTUEEE Preparation
It is highly recommended that candidates should make their strategy and start preparations to cover the enormous syllabus. Here we have some useful preparation tips to help you crack the exam in the best possible way.
- The VTUEEE Syllabus is based on three subjects, i.e., Physics, Chemistry, and Mathematics of classes 11th and 12th. Hence, it includes chapters that you had in Class XI, as well as Class XII. Revise from your old school books.
- Make a proper study plan and write it down on paper and keep it in sight. Allocate your study hours/days to different topics and ensure that the preparation of each topic gets completed as per the time assigned. It is the only way to cover the syllabus if the candidate starts studying within an adequate time.
- Revision leads to better learning. That's why you should revise every day because, without this, you may not remember whatever you study.
- Get your basics clear. As per the standard of the entrance exam in the last few years, candidates are expected to answer conceptual questions for which MCQ type questions will be provided.
- Refer to the previous year's question papers of the VTUEEE exam and solve mock tests during the last few weeks before the exam. This will enhance the time management competency and help the candidate.
VTUEEE FAQs
Q1. Will the application fee be refunded in case I do not give the examination?
A1. No, the application fee as per the policy of the institute is cannot be refunded in any case. There are no exemptions.
Q2. Is there any system for negative marking in the VTUEEE 2021?
A2. No, there isn't a system for negative marking in the examination.
Q3. Will I be able to pay the application fees through net banking or in any other online method?
A3. Yes, candidates will be allowed to submit the application fees through debit cards, credit cards, and net banking also.
Q4. Can the application form be filled in offline mode?
A4. The application form is made available both in online and offline mode and hence you can fill the form in offline mode also.
Q5. Where can I fill my exam city preferences?
A5. You will be able to fill in three exam city preferences at the time of registration.
Best Wishes to all VTUEEE aspirants from the CollegeMarg Team!












